Technology: Electrolyser
When employing renewable energy sources for water electrolysis it is vital to prevent electrode degradation induced by fluctuating energy input. Mechanical stability and process efficiency suffer when input power varies. The lifetime of standard electrodes in this operational mode only lasts a few hours. For this reason electrolysers are conventionally operated at constant rated power. During shutdown, standard electrodes will corrode unless a protective voltage is applied. This requires an electricity backup and causes energy losses.
The objective of the EUHYFIS research was to find improved electrode materials. Such innovative electrodes were subjected to severe conditions of operation for more than 1,200 hours in a 5 kW test rig and displayed an excellent stability. After more than 3,000 total current interruptions and a large number of strong power fluctuations from a wind energy input simulator, no deactivation was observed. No protective voltage was required to stabilise the electrodes, either.
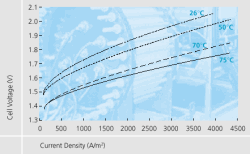
Furthermore, the newly developed electrodes display excellent current density – cell voltage characteristics. At temperatures of 75°C and 4000 A/m2 , for example, the cell voltage is 1.75 V. Under the same circumstances standard electrodes exhibit about 2.1 V. Process efficiency was thus improved by 15 %.
These advantages persist at low electrolyser temperatures which will occur when restarting the unit from stand-by or in part-load operation. Even at 26°C (and 4000A/m2 ) the cell voltage remains below 2.1 V.
The energy required for producing 1 Nm3 of hydrogen with the new-generation electrodes is 4.2 kWh at full load (4000 A/m2 , 75°C). The corresponding process efficiency relative to the gross / net calorific value of hydrogen amounts to 84.5 % and 71.3 %, respectively. Purity is higher than 99.8 % by volume and can be increased to 99.999 % where necessary. Output pressure of the hydrogen gas ranges from 6 to 30 bar.